P V And T V Diagrams For Water

P x y and t x y diagrams for vapor liquid equilibrium vle rachael l.
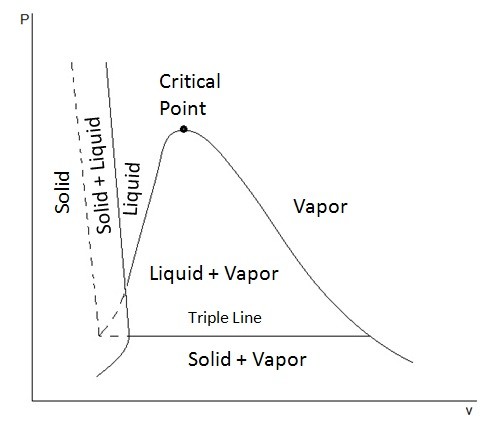
P v and t v diagrams for water. No air in this system air is a different system. O 2 n 2 t 273 16 k p 0 6113 kpa p 0 00602 atm. Bozeman science 102 999 views 7 53 115 videos play all egr 263 thermodynamics. We also know that the area 12 ba in the t s diagram represents the heat absorbed to some scale during the isothermal expansion.
The figures below show the p v diagrams for substance that has its solid phase contract during freezing and the p v diagram for a substance that has its solid phase expand during freezing. Thermodynamics and p v diagrams duration. This video explains how to draw t v diagrams and what they represent. The curve on the p v and t v diagrams in the solution is the saturation dome for water.
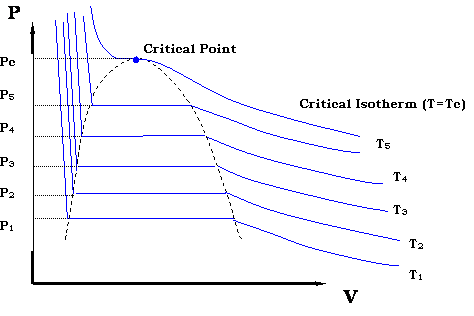
100 c 500 kpa b ammonia. This isothermal expansion is represented by curve 1 2 on p v and t s diagram in the figure. Baumann construct a t x y diagram for vapor liquid equilibrium vle. P v and t v diagrams can also show the phase changes between all three phases.
The p v diagram for water the above discussion was done in terms of the t v diagram however recall from chapter 1 when we defined the state postulate that any two independent intensive properties can be used to completely define all other intensive state properties. We know that the heat is absorbed by water during its conversion into dry steam is its latent heat l1. Along triple line where t p fixed for range of v. On the saturation dome water is a saturated vapor.
Baumann adding a second component to a single component vapor liquid equilibrium vle mixture rachael l. Below it water is a liquid. 10 c 150 kpa c r 410a. All 3 exist only at the triple t p ice s water l water v note.
Above it water is a vapor steam.